Abstract
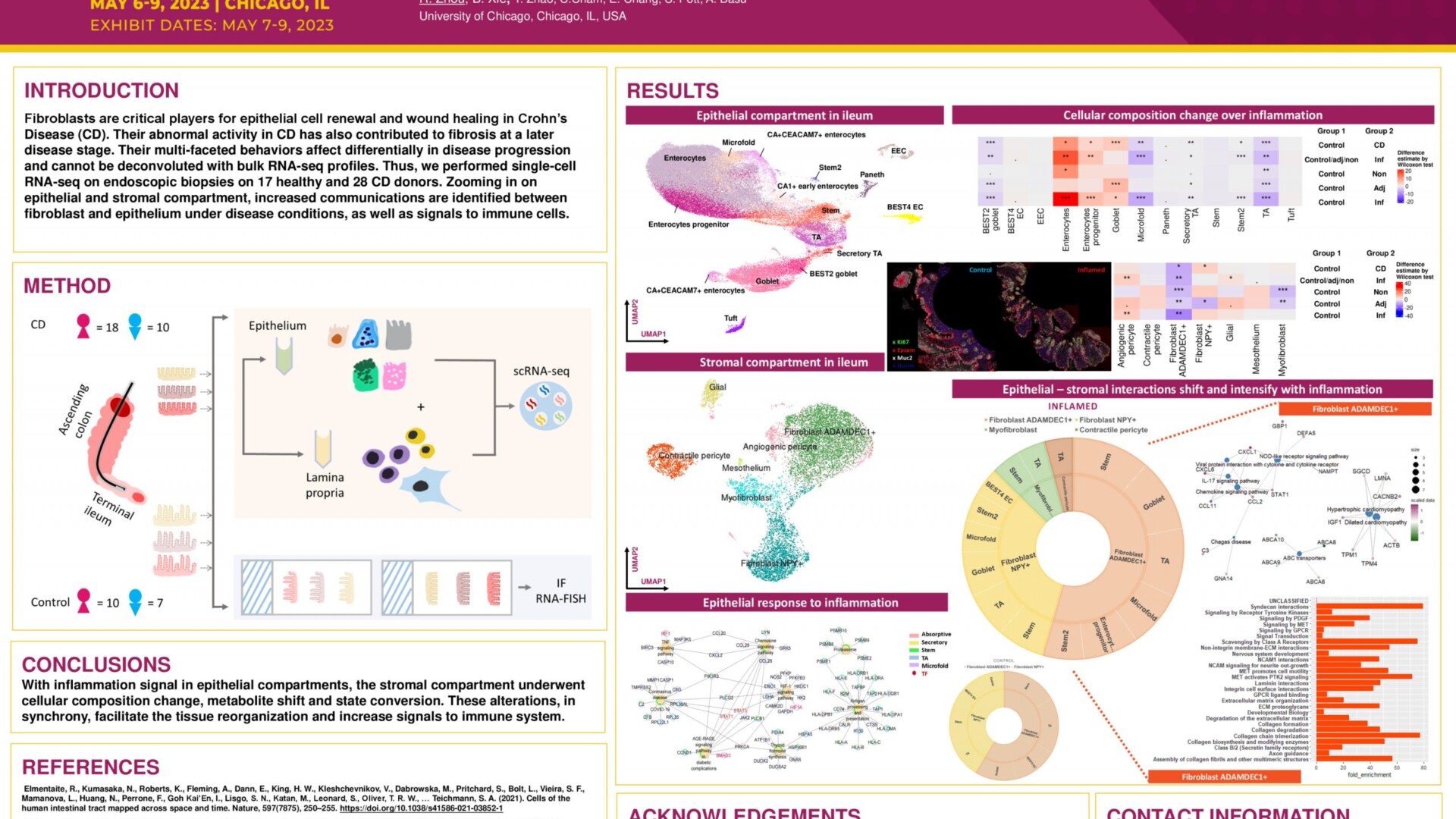
Background: A major impact of the microbiome on gut physiology is via the generation of bioactive metabolites and small molecules. In recent years, the realization that gut microbiota-generated metabolites can regulate signaling pathways in multiple organ systems has prompted interest in investigating the molecular mechanisms whereby these small molecules function. Employing advanced methodology to maintain axenic and gnotobiotic mice, and mass spectrometry-based metabolomics platforms for analysis of small molecules, our research group demonstrated remarkable differences in the metabolite composition of the mitochondria in germ-free and conventional mice. The most discriminatory metabolite generated by the microbiome was δ-valerobetaine (VB). Our previous studies showed that VB suppresses mitochondrial fatty acid oxidation in hepatic cells by decreasing cellular carnitine levels. We showed that VB is a central integrator whereby the microbiota influences energy metabolism is host tissues. Hypothesis: VB derived from the microbiome can influence mitochondrial bioenergetics in cells within the intestinal epithelium, thereby impacting intestinal cell homeostasis and gut epithelia barrier integrity. Methods: Germ-free mice were treated intraperitoneally with VB or vehicle control and upon sacrifice, the colon and small intestine were removed. Swiss rolls were made from both colon and small intestine and stained for proliferative and mitochondrial markers to assess the effect of VB on proliferation in the cell crypt and mitochondrial expansion. Furthermore, tissue samples were analyzed to assess whether VB alters the expression of markers of mitochondrial biogenesis markers and gut epithelial integrity. In addition, VB was administered to the media of Caco-2 cultured cells to determine the effect of VB on mitochondrial biogenesis. Results: Germ-free mice treated with 50µM of VB for 4 days had increased mitochondrial abundance (TOM20 staining) in the small intestine compared to vehicle treated control mice. Furthermore, VB treated germ-free mice had increased numbers of proliferating cells in the small intestinal crypts (quantifying Ki-67 positive cells). VB-treated mice also had a significantly higher expression of the regenerative stem cell markers Ly6A and Clu. Conclusions: Our results suggest that the novel microbiome generated metabolite, VB can induce mitochondrial biogenesis in the intestine, thereby implicating VB as an integrator of host cell and microbe interactions in intestinal epithelial homeostasis.